Label the image of the bomb calorimeter, a captivating exploration into the intricacies of this scientific apparatus, unveils a world of precision and discovery. This comprehensive guide delves into the intricacies of the bomb calorimeter, providing a thorough understanding of its components, operation, and applications.
As we embark on this journey, we will meticulously examine the bomb calorimeter’s diagram, deciphering the functions of each component. We will then delve into the step-by-step procedure for operating this instrument, ensuring accurate and reliable measurements. Furthermore, we will explore the intricacies of data analysis, unraveling the calculations that unveil the heat of combustion.
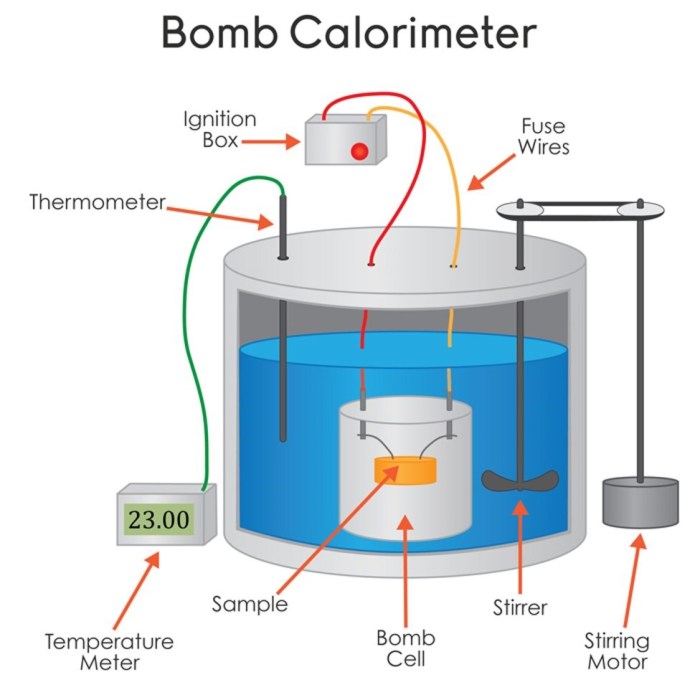
1. Bomb Calorimeter Diagram
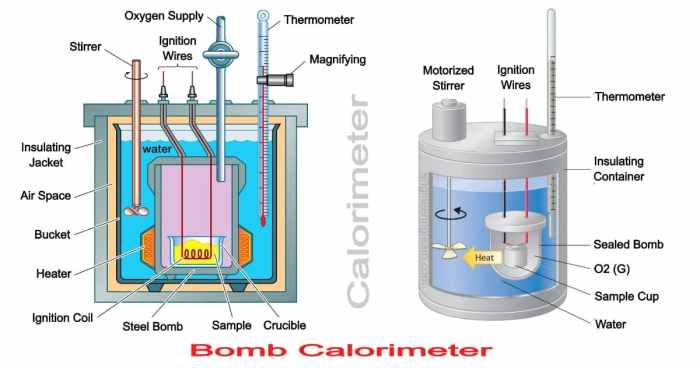
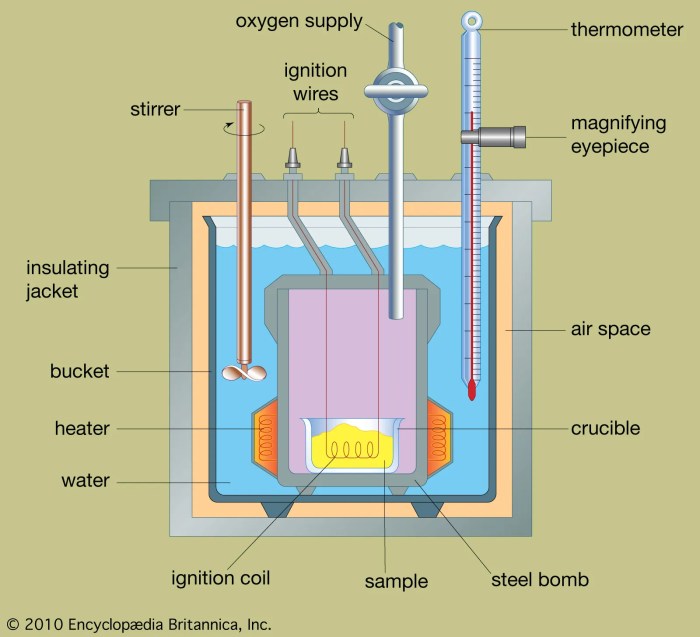
A bomb calorimeter is a device used to measure the heat of combustion of a substance. It consists of a strong, sealed container (the bomb) that holds the sample, a known amount of water, and an oxygen atmosphere. The bomb is placed in a water bath, and the temperature of the water is measured before and after the combustion reaction.
The heat released by the reaction is calculated from the change in water temperature and the heat capacity of the calorimeter.
Diagram of a Bomb Calorimeter, Label the image of the bomb calorimeter

The major components of a bomb calorimeter are labeled in the diagram:
- Bomb
- Water bath
- Stirrer
- Thermometer
- Oxygen inlet
- Ignition wire
- Pressure gauge
2. Operating Procedure
The step-by-step procedure for using a bomb calorimeter to determine the heat of combustion is as follows:
- Prepare the sample by weighing it and placing it in the bomb.
- Fill the bomb with oxygen to a pressure of about 30 atmospheres.
- Place the bomb in the water bath and stir the water.
- Ignite the sample by passing an electric current through the ignition wire.
- Record the temperature of the water before and after the combustion reaction.
- Calculate the heat of combustion from the change in water temperature and the heat capacity of the calorimeter.
3. Data Analysis
The data obtained from a bomb calorimeter experiment are used to calculate the heat of combustion of the sample. The heat of combustion is the amount of heat released when one mole of the substance is burned completely in oxygen.
The heat of combustion is calculated using the following equation:
“`Q = m
- C
- ΔT
“`where:* Q is the heat of combustion (in joules)
- m is the mass of the sample (in grams)
- C is the heat capacity of the calorimeter (in joules per gram per degree Celsius)
- ΔT is the change in water temperature (in degrees Celsius)
4. Sources of Error
There are several potential sources of error in bomb calorimetry experiments. These include:
- Incomplete combustion of the sample
- Heat loss to the surroundings
- Calibration errors
- Errors in measuring the mass of the sample and the water
- Errors in measuring the temperature of the water
These errors can be minimized by carefully following the operating procedure and by using accurate equipment.
5. Applications
Bomb calorimetry is used in a variety of fields, including:
- Food chemistry
- Materials science
- Environmental studies
In food chemistry, bomb calorimetry is used to determine the energy content of foods. In materials science, bomb calorimetry is used to determine the thermal properties of materials. In environmental studies, bomb calorimetry is used to determine the heat of combustion of fuels and other materials.
Answers to Common Questions: Label The Image Of The Bomb Calorimeter
What is the purpose of a bomb calorimeter?
A bomb calorimeter is a device used to measure the heat of combustion of a substance. It is used in various fields, including food chemistry, materials science, and environmental studies.
How does a bomb calorimeter work?
A bomb calorimeter consists of a sealed container filled with oxygen. The sample is placed inside the container, and the container is then submerged in a water bath. The sample is ignited, and the heat released during combustion is absorbed by the water.
The temperature change of the water is measured, and this data is used to calculate the heat of combustion.
What are the applications of a bomb calorimeter?
Bomb calorimeters are used in a variety of applications, including:
- Determining the energy content of foods
- Studying the thermal properties of materials
- Measuring the heat of combustion of fuels
- Calibrating other calorimeters